This site uses cookies for analytics and to improve your experience. By clicking Accept, you consent to our use of cookies. Learn more in our privacy policy.
This webinar was specifically designed and delivered for the members of the British In Vitro Diagnostic Association (BIVDA), in response to their request for guidance on this topic. It highlights how to select an LSP, an individual translator, or a team of translators, explores the hidden challenges of translation, and covers much more.




Medical device translations often involve hidden challenges such as unclear source text, missing references or glossaries, limited context, vague briefs, unrealistic timelines, and conflicting reviewer feedback—all of which can impact quality and compliance.
With over 700,000 translators worldwide, verifying expertise, quality, and reliability is difficult. Many companies lack vendor management and QA structures, while online platforms often offer no ISO workflows, terminology control, accountability, or risk management.
Working effectively with translators means sharing purpose and context, providing clear briefs and terminology early, keeping communication open, and respecting essential quality steps. Timely client feedback directly impacts quality and delivery.
Effective collaboration with LSPs relies on life-sciences expertise, ISO-aligned workflows, clear roles and communication, strong terminology and version control, a transparent AI/MT policy, and full process and pricing transparency.
The 2026 Planning & Strategy for Medical Device Manufacturers online workshop has been completed and focused on real-world, operational challenges related to multilingual medical device documentation, regulatory readiness, and long-term scalability.
Selected highlight videos from the workshop are available below, followed by an overview of additional key topics discussed during the session.



Why unclear or incomplete briefs are one of the most common causes of delays, rework, and quality issues and what information should be included from the outset to ensure smoother workflows.
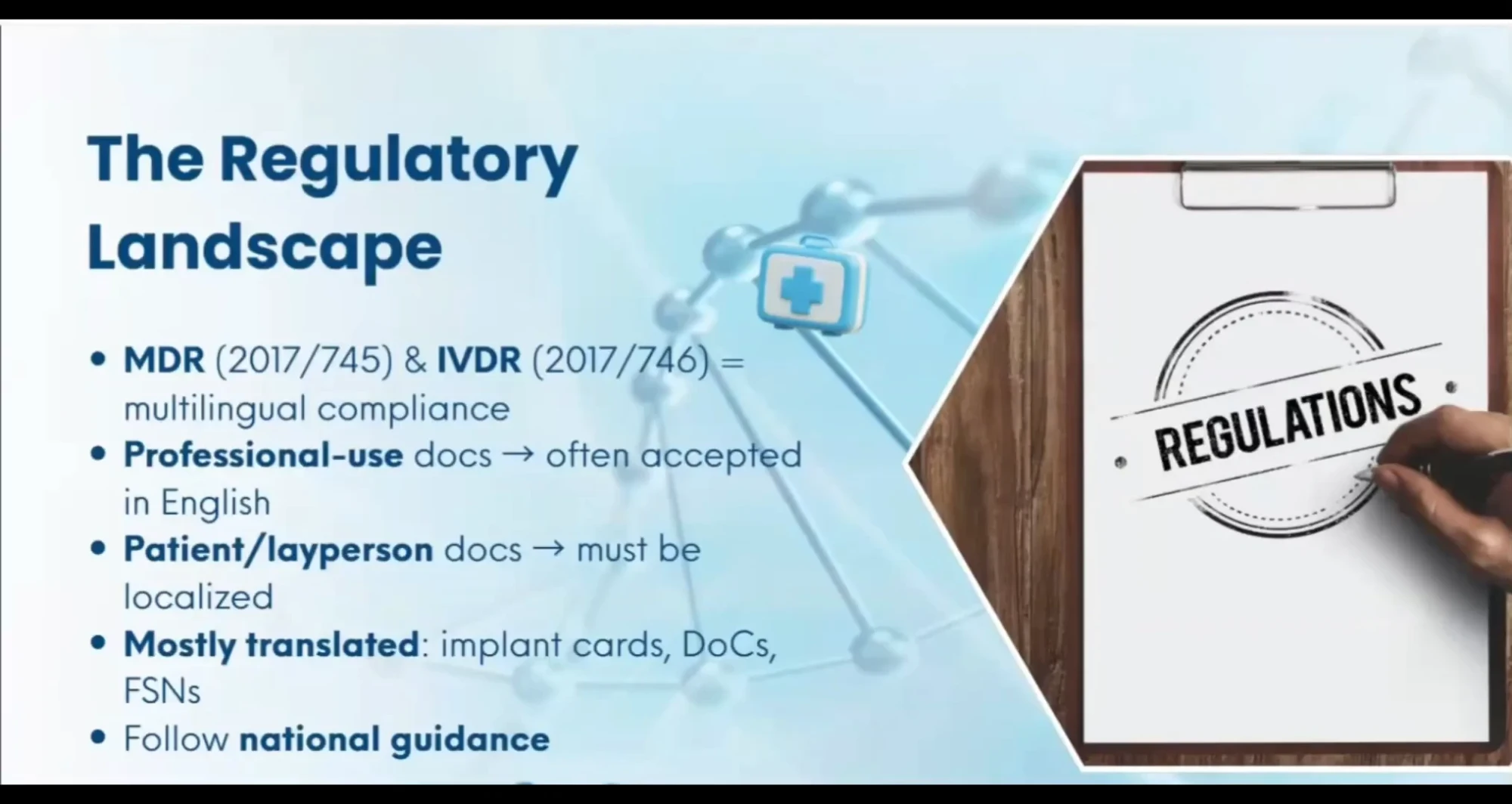
An in-depth look at the disconnect between regulatory acceptance of English-only IFUs and the practical challenges encountered during actual market sales, device deployment and distributor use.
Who should own translation memories and terminology assets and how ownership impacts consistency, compliance, scalability, and long-term cost control.
How to involve distributors in the translation process without losing control, consistency, or regulatory alignment.
Would you like to learn how to master MDR/IVDR translation compliance with confidence?
You can now watch a selected excerpt from our Tremédica webinar:
“Excellence in Medical Device Translations: Mastering Regulatory Compliance Without Fear.”



We are a specialized life sciences translation and business outcomes partner supporting medical device manufacturers with precise, regulator-ready translations across global markets.
Our workflows are built around ISO-certified processes, experienced medical linguists, and multi-step quality controls to ensure accuracy, consistency, and traceability.
We help you move confidently through MDR, IVDR, and international regulatory requirements by aligning language, documentation, and terminology with current expectations
Every translation is handled with patient safety, risk mitigation, and regulatory compliance in mind because in medical devices, words matter.
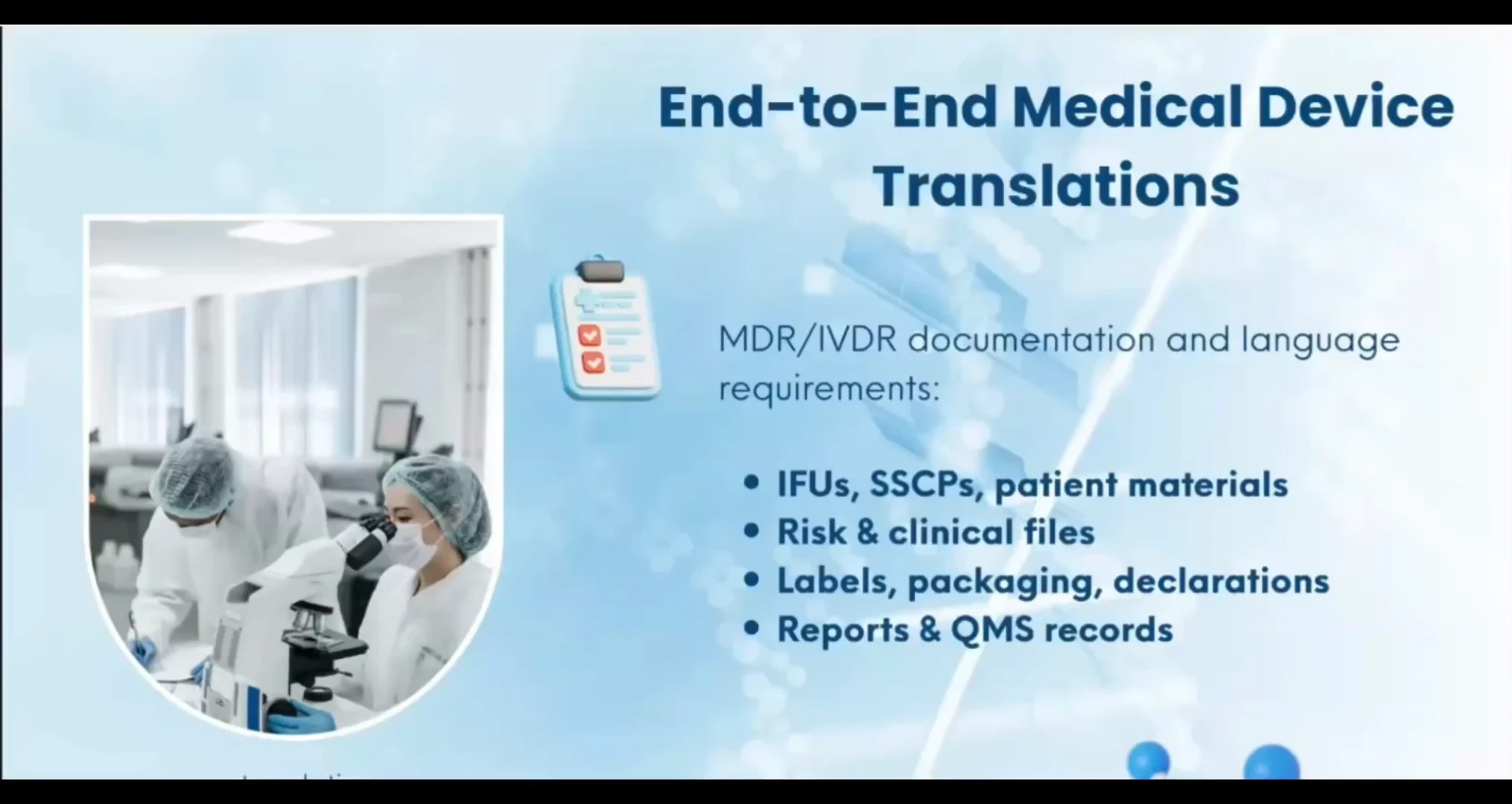
We deliver complete medical device translation solutions from instructions for use and labels to technical files managed seamlessly from start to finish.