This site uses cookies for analytics and to improve your experience. By clicking Accept, you consent to our use of cookies. Learn more in our privacy policy.

I’m Nataliya Nedkova, a life sciences translator, linguistic validation consultant, and the founder of NN Translations — an ISO-certified content and business outcomes provider I established in 2010 to bring more care, clarity, and regulatory precision to medical and technical documentation.
Over the years, I’ve specialised in medical device translations, regulatory documentation, and linguistic validation for clinical trials, always guided by one core principle: ensuring regulatory compliance, conceptual clarity, and patient safety.
Today, together with my dedicated team, I support MedTech companies worldwide — medical device manufacturers, pharmaceutical companies, CROs, and eCOA providers in more than 180 languages.
Our services are certified under ISO 9001 and ISO 17100, and aligned with ISO 13485, ensuring every workflow is structured, traceable, and fully auditable to meet international quality and regulatory expectations.
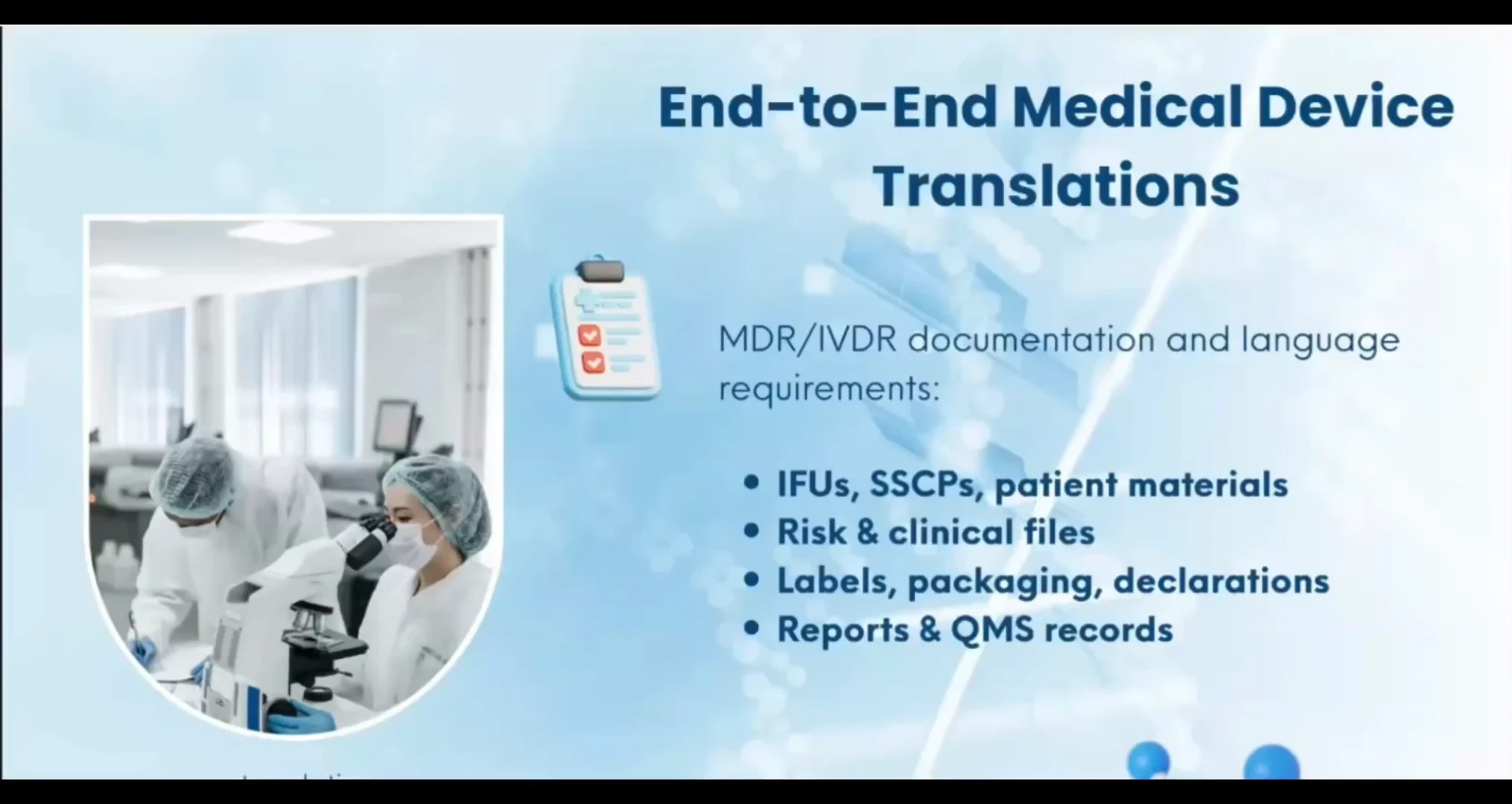
At NN Translations, we focus on medical device, pharmaceutical, and life-science documentation — everything from MDR/IVDR-compliant translations, IFUs, leaflets, and SSCPs to strategic advice on regulatory affairs, compliance, legal, marketing, and distribution through our network of trusted consultants.
This enables us to offer clients a complete solution under one roof, supporting not only their documentation needs but also the broader strategic requirements that influence successful submissions.
Our main role is to help manufacturers prevent delays during submissions by ensuring their documentation is accurate, consistent, and fully aligned with regulatory, legal, and technical requirements.
Because we support around 180 languages, clients rely on us when they need to launch products or submit documentation across multiple EU and global markets — confidently and on time.
Whether you require MDR/IVDR-compliant content, multilingual alignment, terminology consistency, or strategic input to strengthen your submission package, we are here to help you move through every stage of the regulatory and post-market process with clarity and confidence.
We understand the challenges MedTech companies face when managing multilingual MDR/IVDR documentation and broader business needs. Here’s how we help you stay compliant, consistent, and fully supported throughout your product lifecycle:
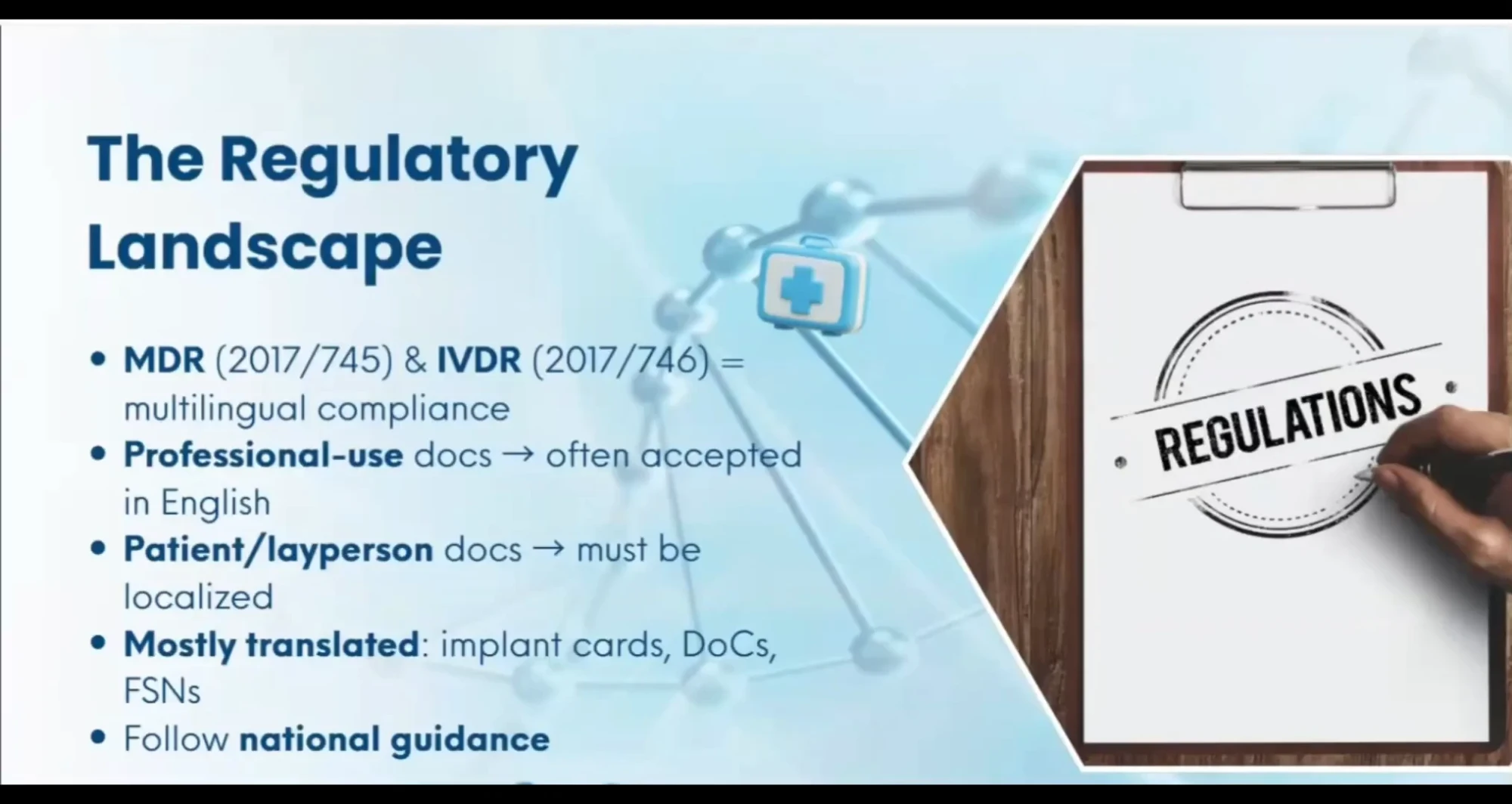
We translate and format documentation according to MDR 2017/745 and IVDR 2017/746, and national language requirements, helping you avoid costly rework, delays, or rejections. Our work fully aligns with MDR/IVDR Article 2 and Annex I (Ch. III), EMDN, GMDN, IMDRF, ISO 20417:2021, the ISO 18113 series (2022), ISO 15223-1:2021, WHO MeDevIS, WHO ICD-11, EDQM, FDA, and other relevant international frameworks.
All linguists are native-speaking subject-matter experts with proven experience in your device subfield – whether diagnostic, dental, or implantable devices.
Our Quality Procedure addresses any cases of poor translation quality or missed deadlines, ensuring full accountability and continuous improvement. Every project includes a translation evidence track ready for audits or Notified Body reviews.
We create and maintain client-specific glossaries, translation memories, style guides and a medical device terminology resource to guarantee accuracy and consistency across all documents and updates.
We don’t just translate — we educate and advise our clients on how to approach each project, which workflow best fits their document type and compliance level, and how to achieve the most efficient, audit-ready outcome.
We tailor each workflow to your document type and compliance level – from translation + revision to in-country or clinician review, ensuring the right balance between efficiency and accuracy.
Our structured project management ensures your translations are ready before submission deadlines, so your regulatory teams can stay focused on strategy and innovation.
Beyond translations, our consultancy teams provide regulatory, compliance, legal, and marketing advice to support your broader business goals. For distribution needs, we connect you with our trusted local network to help you navigate market entry and regional requirements. This integrated approach helps your company thrive — ensuring not only compliant documentation, but stronger outcomes across the entire product lifecycle.
We’ve developed a complete set of internal and client-facing documentation because we’re thinking about you – your needs, your challenges, and the peace of mind you deserve when partnering with a life sciences content and business outcomes solutions provider.
Each document is designed to make our collaboration transparent, structured, and aligned with your regulatory and quality goals.
Before each project, we use a Pre-Project Translation Qualifying Questionnaire to understand your requirements in detail and identify potential risks early. This ensures that every aspect – from scope and terminology to timelines and deliverables – is clear from the start.
For us these materials are more than documents – they reflect our commitment to serve you with clarity, structure, and care, helping you achieve compliance and quality excellence without stress or uncertainty.
If you want me to advise you on your process or fix problems you are experiencing, just click below to schedule a 15-minute introductory call with me.
Would you like to learn how to master MDR/IVDR translation compliance with confidence?
You can now watch a selected excerpt from our Tremédica webinar:
“Excellence in Medical Device Translations: Mastering Regulatory Compliance Without Fear.”